Identify the Electrophile in a Friedel-crafts Alkylation
A Friedel-Crafts alkylation reaction is an electrophilic aromatic substitution reaction in which a carbocation is attacked by a pi bond from an aromatic ring with the net result that one of the aromatic protons is replaced by an alkyl group. Similar to the FriedelCrafts acylation the electron-donating groups facilitate the alkylation whereas the electron-withdrawing groups impede the alkylation.

Solved Identify The Electrophile In A Friedel Crafts Chegg Com
A carbocation is the electrophile of this reaction and is generated by protonating the tertiary alcohol.
. The nucleophile of this reaction is the 14-dimethoxybenzene which attacks the electrophile and creates a resonance stabilized. Carbocation reacts as electrophile in Friedal craft alkylation reaction. Addition of electrophile loss of proton E.
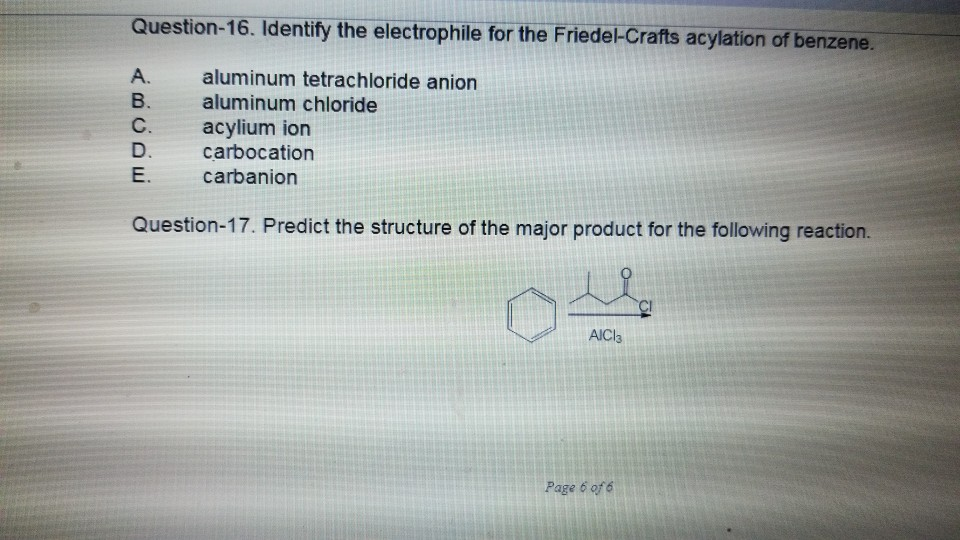
The goal of the reaction is the following. Asked Aug 25 2019 in Chemistry by Janelle. RCO RCO acylium ion AlCl4.
Identify the electrophile in a Friedel-Crafts alkylation. The electrophilic bromination or chlorination of benzene requires _____ along with the halogen. The purpose of this experiment is to make a dialkylated product using an unknown tertiary alcohol and 14-dimethoxybenzene via Friedel-Crafts alkylation.
A Lewis acid is a molecule with an empty orbital that can accept an. Acylium ion does not undergo rearrangement. Diferença principal - Friedel Crafts Acylation vs Alkylation.
Aryl and Vinyl halides cannot be used in the Friedel-Crafts alkylation reaction because they do not _____. View the full answer. Aluminum chloride carbocation aluminum tetrachloride ion carbanion radical.
A FriedelCrafts Alkylation is an electrophilic aromatic substitution in which the Electrophile E is a carbocation. Friedel-Crafts acylation follows a similar mechanism as the alkylation with the first step being activation of the acyl halide. Rearrangements in Friedel -Crafts Alkylation Isobutyl chloride is the alkyl halide.
อะไรคอความแตกตางระหวาง Friedel Crafts Acylation และ Alkylation acylation ของ Friedel แลกเปลยนกลมของ acyl ในขณะท alkylation ของ Friedel Crafts. The Acylium Ion is the Electrophile in Friedel-Crafts Acylation Reactions. To overcome this a strong Lewis acid is used to help catalyse the reaction.
In previous chapters we have seen other methods of forming carbocations such as protonation of an alkene using a Strong AcidThe resulting carbocation can also be attacked by a benzene ring resulting in alkylation of the aromatic ring. Friedel-Crafts Alkylation Pre-Lab katie hudson keh25506 fall 2021 chem 2212l november 15 2021 alkylation introduction the purpose of this experiment is to. The resulting product places a carbonyl group next to the aromatic ring.
Identify the electrophile in a Friedel-Crafts alkylation. A Friedel-Crafts alkylation is an electrophilic aromatic substitution in which the electrophile E is a carbocation. But tert-butyl cation is the electrophile.
An alkyl group can be added to a benzene molecule by an electrophile aromatic substitution reaction called the FriedelCrafts alkylation reaction. The FriedelCraft alkylation is a special class of electrophilic aromatic substitution in which the electrophile is a carbocation. The alkyl halide reacts with the Lewis acid to form a a more electrophilic C a carbocation.
MECHANISM FOR THE FRIEDEL-CRAFTS ALKYLATION OF BENZENE. However benzene is not a very good nucleophile and cannot react directly with the alkyl halide. In the Friedel-Crafts alkylation reaction the benzene ring acts as a nucleophile and an alkyl halide acts as an electrophile.
Identify a limitation of the Friedel-Crafts alkylation of benzene. Loss of the halide to the Lewis acid forms the electrophilic alkyl carbocation. The electrophilic species in a Friedel-Crafts alkylation reaction depends on the identity of the alkyl halide.
Asked Jun 15 2017 in. The very first step involves the formation of the acylium ion which will later react with benzene. In previous chapters we have seen other methods of forming carbocations such as protonation of an alkene using a strong acid.
The mechanism for this reaction begins with the generation of a methyl carbocation from methylbromide. The active electrophile for Friedel-Crafts Acylation is the acylium ion How does it form. A acilação e alquilação de Friedel Crafts são dois tipos de reações químicas que foram introduzidas pela primeira vez pelos dois cientistas Charles Friedel e James Crafts.
Aluminium trichloride AlCl 3 is often used as a catalyst in Friedel-Crafts reactions since it acts as a Lewis acid and coordinates with the halogens generating an electrophile in the process. With methyl and 1 alkyl halides the electrophile is a ______ while for 2 and 3 alkyl halides the electrophile is a ______. Aluminum tetrachloride ion B.
One example is the addition of a methyl group to a benzene ring. 2 H2O 1 AlCl3. Friedel-Crafts Alkylation refers to the replacement of an aromatic proton with an alkyl group.
Any electeophile species is that which requires electron pair or may say ele. CH 3 2 CHCH 2 Cl AlCl 3 Isobutyl chloride tert. What is the electrophile in the Friedel-Crafts alkylation reaction with tert-butylchloride.
In addition an acidic catalyst either a Brønsted. Form stable carbocations Identify the electrophile for the.

Solved Question 16 Identify The Electrophile For The Chegg Com
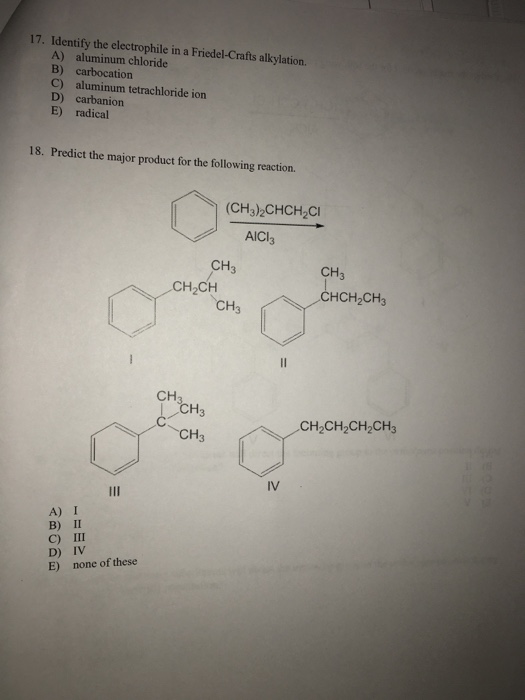
Solved 17 Identify The Electrophile In A Friedel Crafts Chegg Com

Solved Testbank Question 023 Identify The Electrophile In A Chegg Com
0 Response to "Identify the Electrophile in a Friedel-crafts Alkylation"
Post a Comment